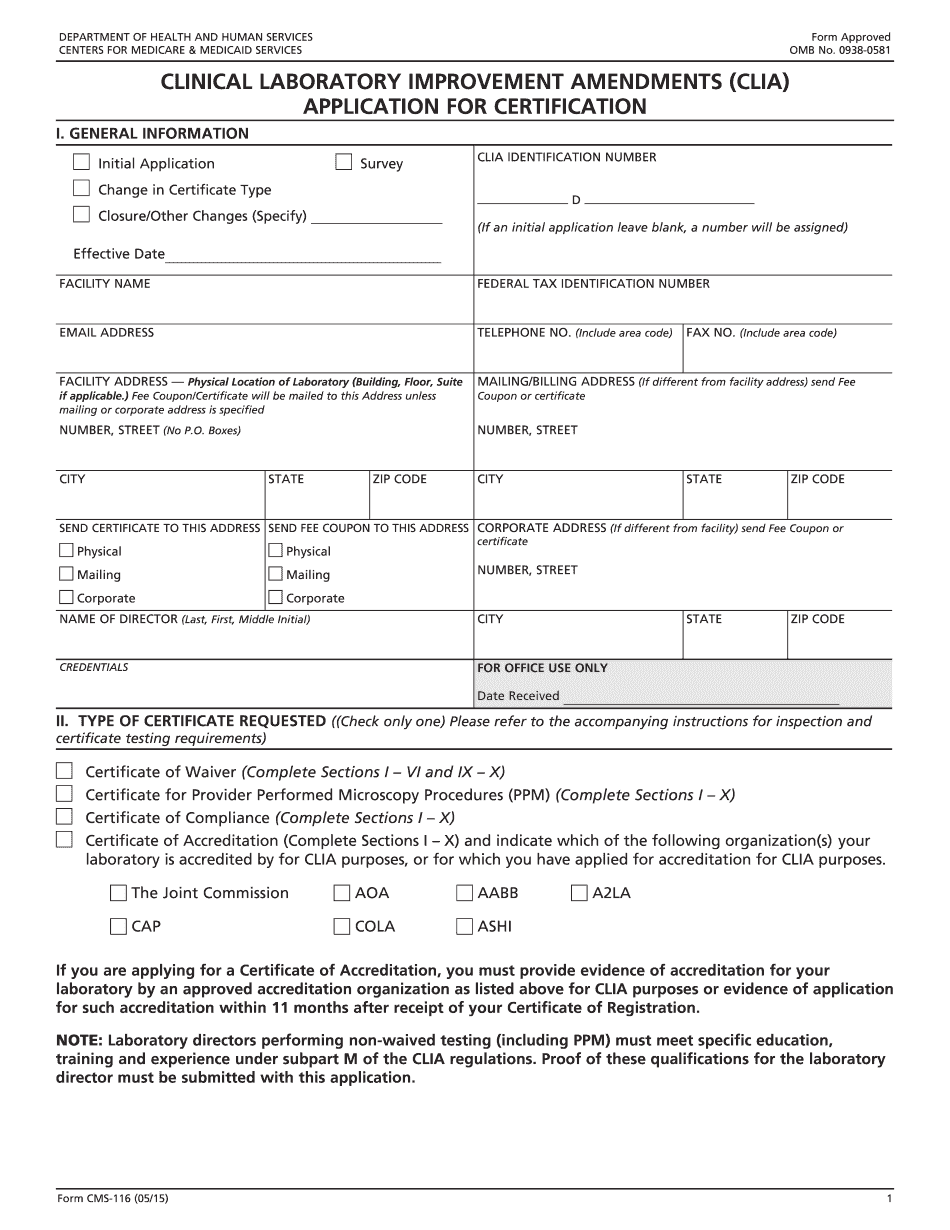
What you should know about CMS-116 Form 2015
- The CLIA Application Form CMS-116 is used to test human specimens for diagnosis, prevention, or treatment purposes.
- Laboratory directors must meet specific education, training, and experience requirements under CLIA regulations.
- Accreditation by approved organizations is necessary for applying for a Certificate of Accreditation.
Award-winning PDF software





How to prepare CMS-116 Form 2015
About CMS-116
CMS-116 refers to the Centers for Medicare & Medicaid Services (CMS) Form 116. It is a document used by healthcare providers, such as hospitals, clinics, and nursing homes, to report statistical information on patient populations, services rendered, and healthcare expenses. This form includes various data elements related to patient demographics, diagnoses, procedures, and utilization patterns. CMS-116 is primarily required for healthcare providers who participate in the Medicare program, which provides health insurance for individuals aged 65 and older, or those with certain disabilities or end-stage renal disease. Providers must submit this form on a regular basis to CMS for the purpose of reimbursement claims, quality assessment, and data analysis. The information collected through CMS-116 helps CMS and other relevant entities monitor healthcare utilization, control costs, evaluate quality of care, and develop policies to improve the healthcare system. It plays a crucial role in the administration and regulation of Medicare services, ensuring accurate and consistent reporting from healthcare providers.