Phlebotomy lesson 1.4 Legal Considerations and Regulatory Agencies. The Occupational Safety and Health Administration, also known as OSHA, has concluded that healthcare workers have an increased risk of occupational exposure to infectious blood and body fluids. This can pose serious health risks because lab personnel routinely work with blood and body fluids and handle sharp objects, unlike other jobs such as a bank teller or a retail sales clerk. Lab personnel have a higher risk of contracting a blood-borne disease. However, this doesn't mean that we need to be afraid. Learning safe practices is an important component of your training program. By learning to your skills safely, you can limit your risk of contracting blood-borne illnesses. There are government agencies that create rules to help you stay as safe as possible. Being alert to danger is a great way to avoid it. OSHA, or the Occupational Safety and Health Administration, is a government agency whose sole purpose is to ensure worker safety. They understand that for-profit employers do not always have the best interests of the workers at heart. Employers may be tempted to limit protective supplies like gloves or sharp containers to increase profits, or they may try to ignore potential dangers so that they don't have to take steps to correct them. As a result, the Occupational Exposure to Blood-Borne Pathogens or BBPS standards were developed. This plan helps set standards for all healthcare employers to follow in order to minimize or eliminate employee exposure to potentially infectious material. For instance, all healthcare employers must provide adequate gloves and other personal protective equipment for their employees. They must educate their employees on the potential dangers in the workplace and develop procedures to minimize those dangers. This is why it is so important that you follow all policies and procedures...
Award-winning PDF software





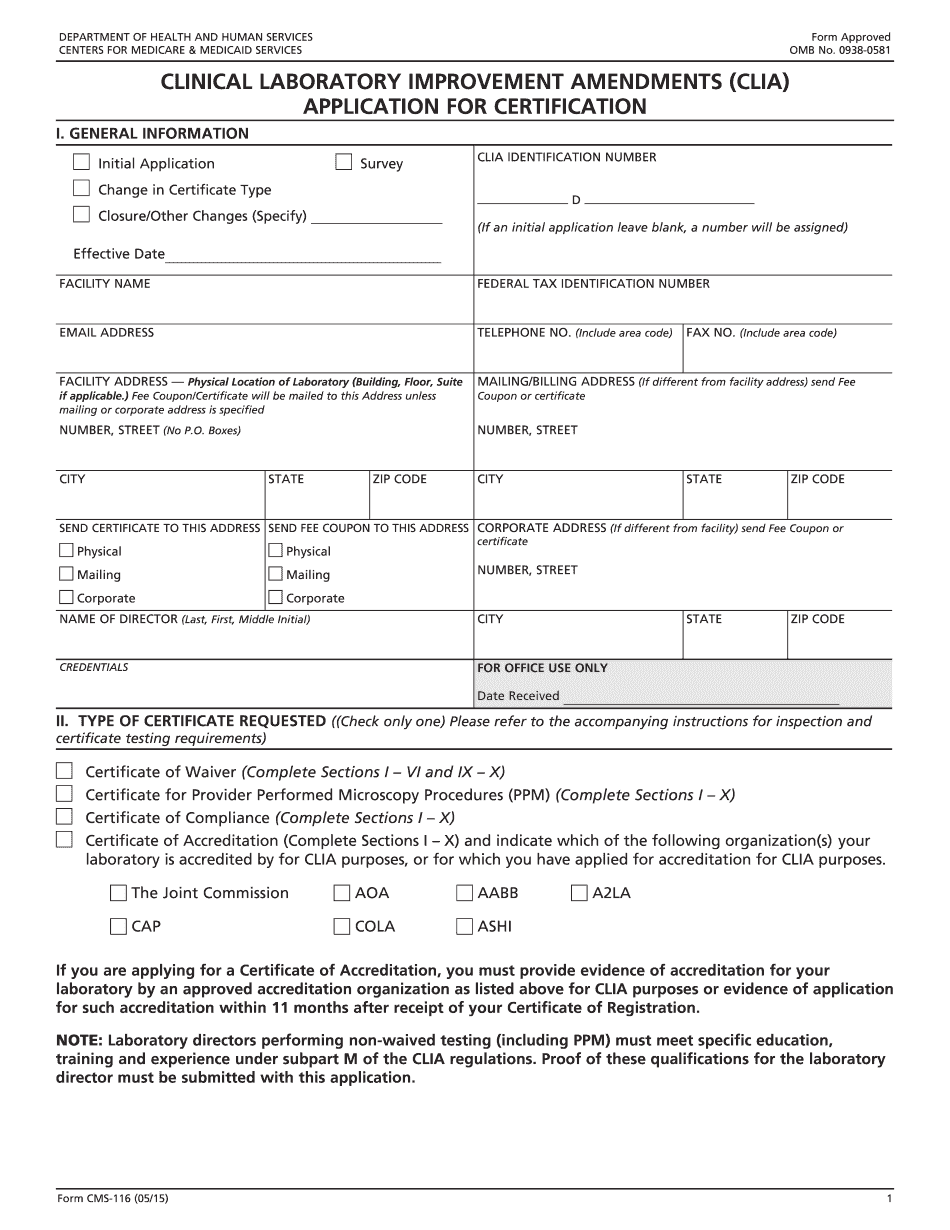
Cms Clia regional office Form: What You Should Know
To report a technical or performance issue within a laboratory, contact the appropriate State Agency (PDF) (click Contact and follow the prompts to find your nearest State Agency) CLINICAL LABORATORY IMPROVEMENT AMENDMENTS OF 1988 (CIA) APPLICATION FOR CERTIFICATION RE: Form #. Your organization has submitted a new, expanded, or amended protocol for NIH approval. The original protocol will not be considered for approval without the additional protocol. This notification will be sent to Clinical and Laboratory Services and the State Agency for the relevant Clinical Laboratory Improvement Amendments (CIA) State Program, and will be attached to the original protocol. This notification will be sent to the address of the State Agency listed on the Clinical Laboratory Improvement Amendments (CIA) State Program Page. This form must be accompanied by an Original Authorization of Certification from the State to be considered for classification. This form must be accompanied by an Original Authorization of Certification from the State to be considered for classification. This form must be accompanied by an Original Authorization of Certification from the State to be considered for classification. To report specific questions or concerns, please contact: Clinical Laboratory Improvement Amendments Clinical Laboratory Improvement Amendments (CIA) State Program Center for Medicare and Medicaid Services — CMS 116 This form is for use only by individuals and organizations located in the United States who are eligible to receive an International Laboratory Improvement Amendments (CIA) Certificate under 5 U.S.C. or have received notification of approval of their State Operations Branch-approved Protocol This form must be accompanied by an Original Authorization of Certification from the State and will be attached to the original protocol. This form must be accompanied by an Original Authorization of Certification from the State to be considered for classification. This form must be accompanied by an Original Authorization of Certification from the State to be considered for classification.
online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do CMS-116, steer clear of blunders along with furnish it in a timely manner:
How to complete any CMS-116 online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your CMS-116 by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your CMS-116 from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.
Video instructions and help with filling out and completing Cms Clia regional office