The Ventana Capellini lab is a certified laboratory for our companion diagnostics life cycle. It is certified by CAP, which stands for College of American Pathologists, and CLIA, which stands for the clinical laboratory Improvement Act. We receive samples from our global pharma trials from the pharma partners that we support in the development of their companion diagnostics. We receive all the tumor samples from the patients that are being considered for enrollment in their trials at the CAT CLIA lab here in Tucson. Their samples are tested by our expert pathologists, who report on the biomarkers being tested. This information helps with enrollment into trials that will be effective for their treatment. The exciting thing about the Cap CLIA lab for Ventana is that every day is a new adventure. Trials begin and end, and new information is gathered. This data, combined with patient outcomes, allows us to make a difference for patients. The constantly changing tests, drugs, and trials make it an exciting environment for the entire team. What excites me most about our CAT play lab are the great people that we have working in the lab. They are highly experienced and have the highest professional integrity. This means that we have a high-quality lab and can produce great data for our pharma partners. Our CLIA certification ensures that the results we provide are the best they can be. This gives me confidence and gives our pharma partners confidence in our work. We attract the best and brightest individuals because we are working at the edge of technology and transforming medicine. We are on the forefront of companion diagnostics and provide solutions to pathologists, oncologists, and patients in their time of need. Despite being highly regulated, we regularly communicate with our pharma partners and have received feedback...
Award-winning PDF software





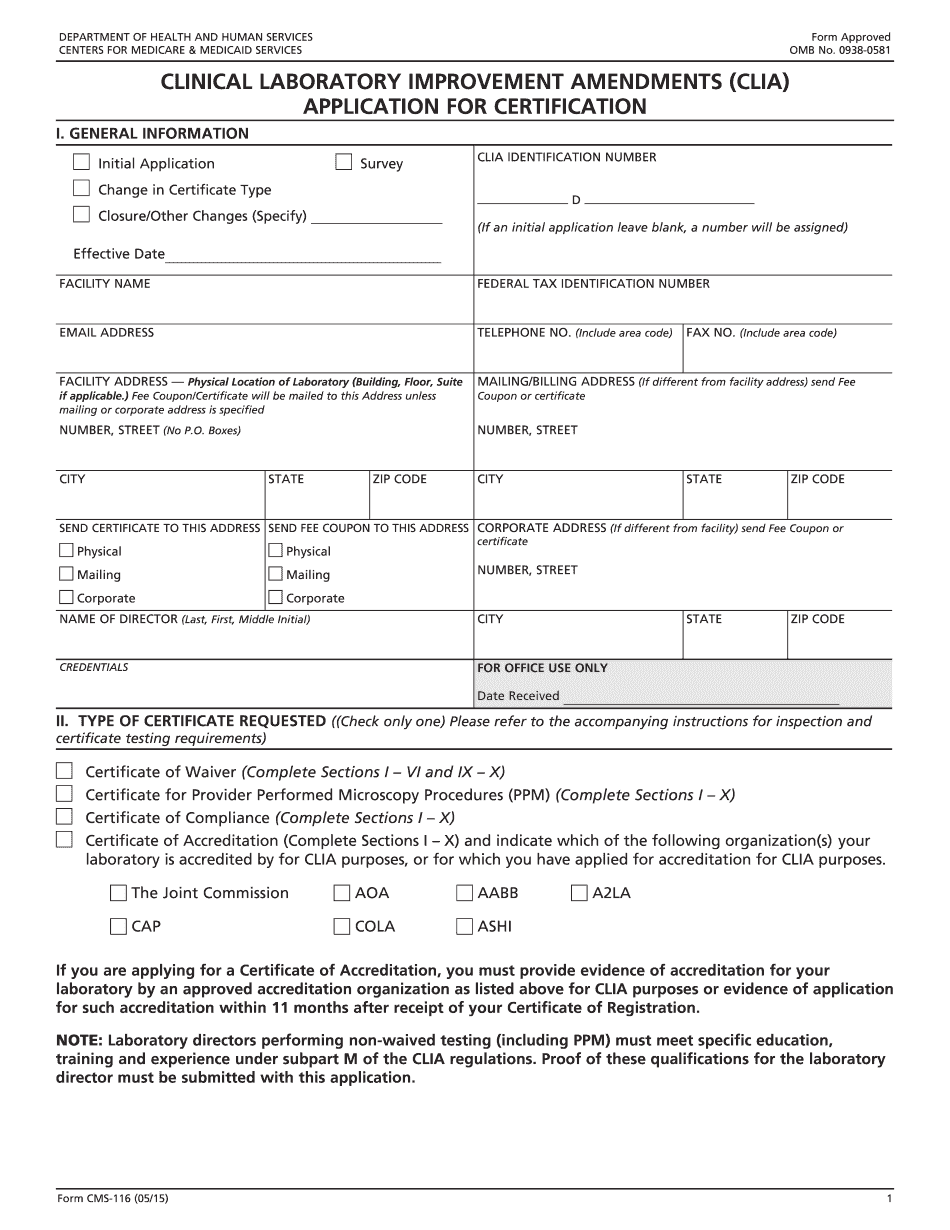
Clia regional offices Form: What You Should Know
OMB No. 0. Joint Certification, Certification for the State Agency. This form must be completed and submitted to the agency located closest to the State in which you presently perform laboratory testing. OMB No. 0. (a) When you are seeking certification for your State Agency for this program, you should: (i) provide appropriate documentation, including letters from the relevant State agency and the American Society of Health-System Pharmacists (ASH-PSP)), which address the issues under consideration in your proposed certification at the time the application is submitted to CMS; (ii) if you are a health facility, report data that support the criteria for eligibility in Section 7B, subparagraph A of the National Health Planning and Resources Act (Title XXVIII of the Social Security Act; 42 U.S.C. 1395 et seq.) for medical home services or hospice, and include the health facility's clinical laboratory data, if available, to support this report within the period of time specified in Section 7A, subparagraph A of the Act. (Note: If you are seeking certification for a health facility with a specialty or specialty group certification, you must submit a separate application and supporting documentation for each specialty or specialty group; see Section 7C, subparagraph C of the Act for further details and to download an additional PDF.) (iii) when processing your application, provide all the supporting documentation referred to in Sections 7B, 7C, and 7D to the applicable State Agency and submit a signed certification that: (A) all the supporting documentation is accurate and complete to the best of your knowledge; and (B) you have no reason to believe that the form is invalid, is incomplete, or contains any errors or omissions. To expedite the certification process, the State Agency may provide the application materials to CMS for review and approval. (iv) Before the agency receives all information necessary to review the application, the application should be submitted electronically (such as by downloading the PDF and emailing it to the agency address above). Once the agency receives all requested information and the submitted application has been reviewed and approved, the State Agency must deliver the certification letter to the applicant. The application for certification should include a statement as follows: “These laboratory standards may change; therefore, information contained in the application should reflect this fact.” Section 7B.
online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do CMS-116, steer clear of blunders along with furnish it in a timely manner:
How to complete any CMS-116 online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your CMS-116 by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your CMS-116 from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.
Video instructions and help with filling out and completing Clia regional offices