All right, so Alicia is going to interview Jane. We're good, we just decided. And this is Boyan, another one of Boyd's great ideas. Is why don't we highlight students so that prospective people in the call can hear how they've used the CCO programs? We don't have a picture, she said. The only thing she had on Facebook was a picture of her cat. So, Jane, let's start out with a little bit of your background. Okay, I trained as a nurse and I went ahead. I was a medical assistant. I had x-ray and lab. And I worked in physicians' offices. And then, as I've got on, I got married, started having children, and I realized that a lot of my time was away from my kids and I didn't like it. So I've always been asked to help in the business office and whatnot. I also have a degree in business from Portland State. And I realized I liked this coding. I liked the insurance part. This looks like fun, but it's a lot of work and a lot of time. And so when I was coding, I just definites said. I'm going to start coding from home. I kind of laughed at, well, what's going to happen? I found in this area, nursing home providers really needed someone to do their billing and their coding, so I started doing that from home. I was able to be around my kids, which was wonderful, and it just really worked out. And then I thought, well, you know, I really should get certified so I know what I'm doing. So I went and I took a course through Portland Community College. And it was at the time when they were just doing online courses, and it was not...
Award-winning PDF software





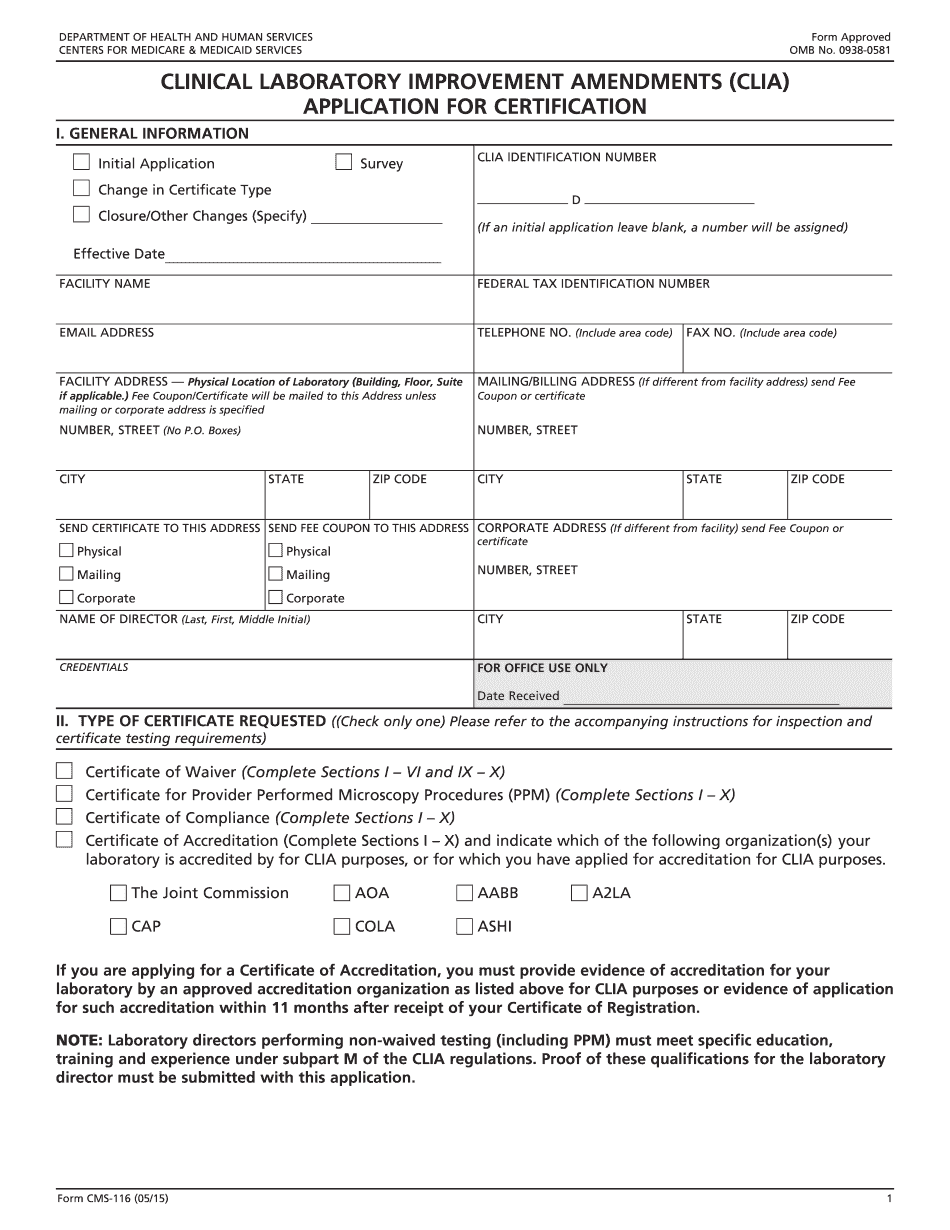
Cms Clia lookup Form: What You Should Know
One cause can be a lengthy investigation done in part by the National Assisting Agency, which can take an indeterminate amount of time. If you are trying to find full time employment, they may choose to wait for further vetting of your history. Second, a person may have received multiple notices from agencies, which can take a number of weeks. Third, if you are hired for a position that does not require a federal background check you may wait longer. However, you are more strongly protected if you have not received any alerts from agencies on your background. Fourth, in the vast majority of cases, one of the following has been the cause for a delayed background check: a delay due to a medical condition or emergency (which must be rectified in that time), The employer has delayed the background check, and has a reasonable expectation that you will not qualify for federal employment (e.g., have a felony conviction) and has made such notification to the background service. Fifth, the background service has delayed the background check because the background investigation is on hold or has been suspended (and there is absolutely no explanation as to why) and the background check is pending. (The agency may still send a notification to you with a “background” status, however, there will be no actual results or decision regarding eligibility until the report or review is complete.) The delays may be caused by many things, including: the investigation is being handled by your local federal agency; the agency is not performing the screening of your records, and there is not a local federal agency performing the screening; the agency is not conducting the background investigation, and there is no local criminal background service that has been requested to do the background investigation; the agency is waiting for the outcome of an investigation or litigation; the agency has received more than one notification of a request for a background investigation, and has sent an alert of every notification, and a delay in the notification of information; or, the agency has received more than one notification of a request for a background investigation and has found that there is a reasonable expectation that you will not qualify for federal employment (e.g.
online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do CMS-116, steer clear of blunders along with furnish it in a timely manner:
How to complete any CMS-116 online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your CMS-116 by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your CMS-116 from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.
Video instructions and help with filling out and completing Cms Clia lookup