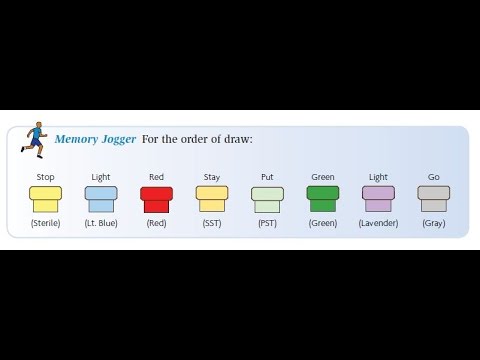
Hi, my name is Al Garza and I'm the creator of Phlebotomy Solutions org. As you can see, I'm sitting here in my office in my house shooting this short introductory video for our YouTube series on phlebotomy. These are PowerPoint presentation videos that I personally use in my lectures when I teach phlebotomy. I've created them to help students prepare for the national state exam, as well as help phlebotomists know more about their craft. I've been an instructor for over 10 years and I've written curriculum for schools throughout the state of California and the state of 64. I have also been in the field of phlebotomy for almost 15 years and have been a teacher for almost 20. These videos are designed to help anyone who is interested in phlebotomy, whether they are taking a phlebotomy course or already working in the field. These volume video DVDs are available on our website, Phlebotomy Solutions org, as well as an instructional hands-on skills DVD called Phlebotomy 101. I would appreciate your input, so please subscribe to our channel and like us on Facebook. You can also visit our personal website, Phlebotomy Solutions org, to let me know what you think. If you would like to see more videos like these or have any suggestions, I can definitely make that happen. So, please check out these videos on our website, Phlebotomy Solutions org. We offer a complete four-volume set and I hope you enjoy them. Please leave your thoughts in the comments below and don't forget to subscribe and like. Thank you and welcome to the Phlebotomy Solutions video series, Volume 4. In this segment, we'll be discussing the order of draw and the additives. The first thing we need to look at are the colors of the tubes....
Award-winning PDF software





Clia list Form: What You Should Know
Listing of Laboratory Employees, Listing of Employees with Individual Accounts, Listing of Individual Employees Who have Direct Account with Clinical Laboratory, Listing of Individual Employees with Shared Account with Clinical Laboratory, Listing of All Applicable SECTIONS of this FORM, Listing of All Applicable SECTIONS of FSA-100, FSA-110, FSA-115, FSA-120, CMR-100-104-105, CMS-105, CMR-115, CMS-130, CMS-120, CMS-130 and FSA-132, CMS-132, CMS-135, CMS-135, CMS-150, CMS-155, CMS-170, CMS-180, CMS-185, CMS-190, CMS-230, CMS-260, CMS-272, CMS-272A, CMS-272B, CMS-272C, CMS-272D, CMS-271M, CMS-271 N, CMS-272O, CMS-272P, CMS-272Q, CMS-292, CMS-295, CMS-297, CMS-298, CMS-300, CMS-400, CMS-410, CMS-441, CMS-448, CMS-460, CMS-461, CMS-463, CMS-467, CMS-472, CMS-474, CMS-476, CMS-478, CMS-479 and CMS-481. CMS-483, CMS-484, CMS-485, CMS-487, CMS-491, CMS-492, CMS-493, CMS-574 and CMS-795. CMS-794. CMS-796 and CMS-798. CMS-206 Clinical Laboratory Improvement Amendments (CIA) This form is to be used in lieu of the CMR-100-104-105 or CMS-105 form if you are only using one (1) of the following forms for your new FDA regulated clinical laboratory or as a stand-alone documentation form. Clinical Laboratory Improvement Amendments (CIA) May 25, 2025 — All Applicable Sections of this FORM MUST be COMPLETED. CMS-116 Clinical Laboratory Personnel Report (CIA) Listing of Personnel by Position by State and Agency. May 25, 2025 — All Applicable Sections of this FORM MUST be COMPLETED. CMS-117 Clinical Laboratory Personnel Report (CIA) Listing of Personnel by Position by State and Agency.
online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do CMS-116, steer clear of blunders along with furnish it in a timely manner:
How to complete any CMS-116 online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your CMS-116 by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your CMS-116 from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.
Video instructions and help with filling out and completing Clia list