Award-winning PDF software





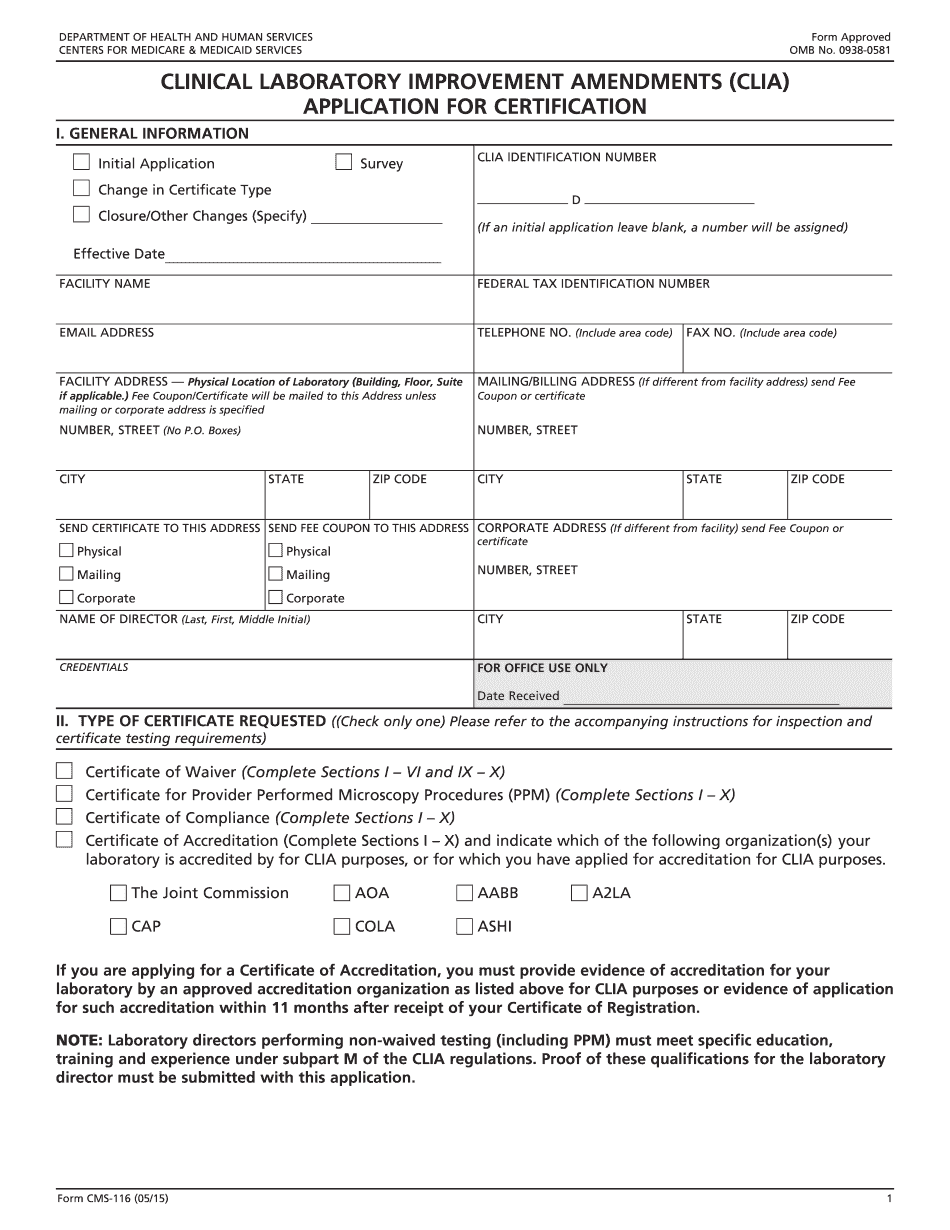
Clia exam Form: What You Should Know
For a certificate, you will need to submit a certificate of compliance, Clinical Laboratory Improvement Amendments (CIA) Reminders— 1. Include the current or estimated annual test volume. 2. The application must reflect the current and or anticipated annual test volumes for your laboratory. 3. If your laboratory does not intend to receive and report the Clinical Laboratory Improvement Amendments (CIA) to CDC, please submit a request to be placed on the FDA's Notice-At-Lobby list. How to Submit Your Certification Application — US Department of Health and Human Services Reminders— For certificate of compliance, submit the application and any appropriate fee, along with certification request Reminder— For certificate of compliance, the application must: include the same information as a Certificate of Compliance that was received from another state in lieu of submitting your current application. Reminder — For an approved device, the application must include: 1. A current listing of ingredients (i.e., allergens) contained in the device. 2. Description of how the device's method of use and labeling comply with the FDA's regulations (i.e., the Device Code). 3. Product or process labeling in compliance with the Food, Drug, and Cosmetic Act (i.e., Labeling Statement or Labeling Statement for Therapeutic Devices). 4. List of all drug-immunoassay test methods in the product. 5. List of all drugs, chemical entities, or other biological products (i.e., components) used in the process (including drug-inactivated components) that are not listed on the product labeling. 6. List of all compounds or components used to make an ingredient of the product (or a substitute for a component) that are not listed on the product labeling and to ensure appropriate control of the use of the ingredients and products in a clinical experiment. For an approved device product labeling: 1. For any product (including those marketed for infants, children, or adolescents) that is approved to meet the requirements of the Food, Drug, and Cosmetic Act (including the Pediatric Device Regulation Act of 2008), the FDA has determined that the labeling meets the criteria in 13 CFR Parts 123 and 145. 2. Use the FDA Common Name/Generic Trade Name of the device or the device name and any combination of the following: FDA common or commercial name, common or commercial name and approved designator number.
online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do CMS-116, steer clear of blunders along with furnish it in a timely manner:
How to complete any CMS-116 online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your CMS-116 by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your CMS-116 from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.